Published on April 26, 2024
Exhibit 99.1
Excerpt
Our Current Pipeline
We have a pipeline of product candidates at various stages of development, including the following:
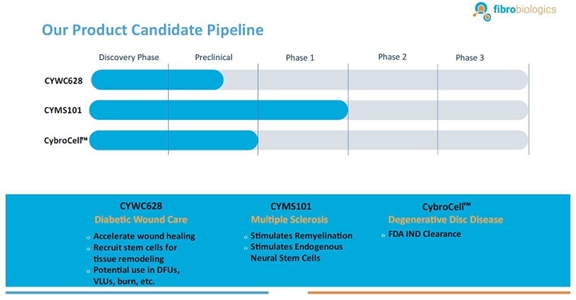
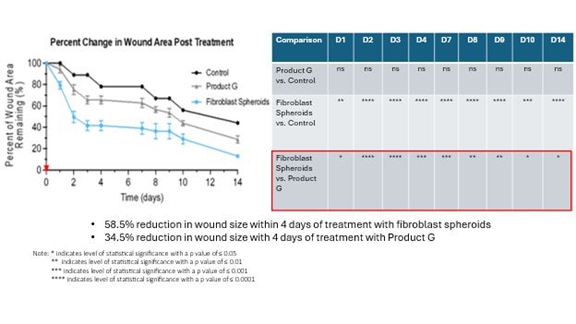
CYWC628 for Wound Healing: We are in the late pre-clinical stages of developing CYWC628 as an allogeneic fibroblast cell-based therapy for wound healing. Our studies are presently focused on utilizing fibroblasts and fibroblast-derived cells to treat wounds in diabetic mice. Our data to date is compiled from four separate animal model studies (manuscript for publication in progress). Each study utilized 16 wild type as well as leptin mutated NONcNZO10LTJ mouse that develops type 2 diabetes when fed a high fat diet. Wound size and area for all our experiments were measured using an eKare inSight™ device which is FDA approved for measuring and monitoring wound size, area and depth. Phase 1 of our pre-clinical study studied the subcutaneous and topically administered single cell mouse dermal fibroblasts (both treatments administered every two days), as well as mouse dermal fibroblast derived exosomes. The results of this study indicated significant improvement in wound healing (p <0.0005) for topically administered mouse fibroblasts and mouse fibroblast exosomes as compared to untreated control, and significant improvement in wound healing with subcutaneous inject of fibroblast in the wound periphery (p < .005). Our Phase 2 pre-clinical study studied the impact of using frozen and thawed single cell mouse fibroblasts administered every two days, as well as mouse spheroid fibroblasts, one-time topical administration, measuring 250 um and each containing approximately 10,000 mouse dermal fibroblasts. In total 100 spheroids were topically administered on to an 8 millimeter diameter wound on the back of the wild type and leptin mutated mice. The results of the study indicated significant improvement in wound healing with the frozen thawed single cell mouse fibroblasts (p < 0.005), as well as 4°C stored mouse fibroblast spheroids (p <0.0005) with both mouse types. Our objective was to test the feasibility of using spheroid fibroblasts as an extended-release mechanism on wound surfaces. The results indicated that spheroid fibroblasts are easier to use and more viable than single cell fibroblasts, and generate more significant results. Our Phase 3 pre-clinical study tested the effect of using a single topical administration of human dermal fibroblast (CYWC628) spheroids compared to a single administration of mouse dermal spheroids, in addition to comparing with a commercially available and FDA approved diabetic foot ulcer treatment called Grafix™. The results of our study indicated that CYWC628 significantly improved wound healing rate (p < 0.0005) as compared to untreated control as well as significant improvement (p < 0.05) over mouse fibroblast spheroids and Grafix™. For our Phase 4 pre-clinical study we studied the impact of a single topical treatment of CYWC628 spheroids and Grafix™ on a chemically induced chronic wound model often used to mimic diabetic foot ulcers in animal models. The results of our study indicated a 58.5% reduction in wound area three days after a single topical administration of CYWC628 as compared to 34.5% for Grafix™ (p < 0.005). The untreated saline control group had an 11% improvement in wound healing which was not statistically significant (p < 0.06). Our results also indicated that with multiple topical administration of CYWC628, the rate of wound closure will likely be more rapid.
The following graph and chart summarize the results of our Phase 4 pre-clinical study.
CYMS101 for Multiple Sclerosis: We are developing CYMS101 as an allogeneic fibroblast cell-based therapy to treat multiple sclerosis, or MS. After completing animal studies using CYMS101 (allogeneic fibroblast cells), we received approval from Mexico to conduct clinical investigations using the fibroblast cell composition for patients with MS and have completed a Phase 1 clinical trial called “Feasibility Study of Tolerogenic Fibroblasts in Patients with Refractory Multiple Sclerosis.” The study was conducted in five participants. The primary objective of the study was to assess safety, and the secondary objective was to assess efficacy. The results of the study for safety were no adverse effects during intravenous injection of the tolerogenic fibroblasts, no short or long-impact in complete blood count test during the 16-week monitoring period, and no short or long impact in electrocardiogram results during the 16-week monitoring period. In addition, the results of the study for efficacy included general improvement of Paced Auditory Serial Addition Test, or PASAT, score for all patients during the 16-week monitoring period, general improvement of 9-hole Peg test completion time for all patients during the 16-week testing period, no general improvement or deterioration noted with the Timed 25-Foot walk test, no general improvement or deterioration noted with Expanded Disability Status Scale, or EDSS, test, and no patient exhibited further deterioration during the trial. We are currently conducting further research to determine the mode of action of fibroblasts in oligodendrocyte expansion and expect to file an IND application for a Phase 2 clinical trial in MS. We will likely seek a strategic partner to collaborate with us on the development of CYMS101 either before initiating the Phase 2 clinical trial, or after its completion, if successful, and prior to commencing with a Phase 3 clinical trial.
CybroCell™ for Degenerative Disc Disease: CybroCell™ is an allogeneic fibroblast cell-based therapy for degenerative disc disease This new technology is being designed as an alternative method for repairing the cartilage of the intervertebral disc (or any other articular cartilage). The method is based on using human dermal fibroblasts, or HDFs, which are forced to differentiate into chondrocyte-like cells in vivo using the mechanical force and intermittent hydrostatic pressure found in the spine, for chondrogenic differentiation of fibroblasts. We believe our solution will prove superior to existing treatments because we expect it will be less invasive, and will regenerate the disc, restore function and reduce pain without debilitating long-term effects. We have completed two rounds of animal studies. The results from the studies were positive and resulted in “first in human” trial approval in our investigational new drug, or IND, submission to the U.S. Food and Drug Administration, or FDA. We have received IND clearance from the FDA, conditional upon approval of our master cell bank, to run a Phase 1/2 clinical trial for patients suffering from degenerative disc disease. We will be conducting this trial within the United States. A timeline will be determined through discussions with the FDA.
| 2 |
Business Update and Recent Developments
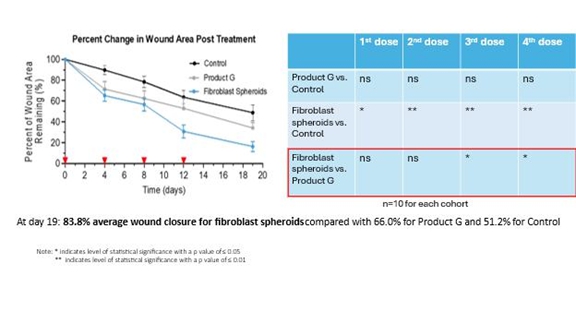
CYWC628 for Wound Healing: For our Phase 5 pre-clinical study, using a diabetic mouse model (BKS.Cg-Dock7m), we studied the impact of multiple administrations of CYWC628 spheroids and Grafix™ on a chemically induced chronic wound often used to mimic diabetic foot ulcers in animal models. The CYWC628 spheroids were administered on Day 0, Day 4, Day 8 and Day 12. The results of our study with this mouse model of a chronic wound indicated (i) a 34.8% reduction in wound area four days after the first administration (day 4) of CYWC628 as compared to 28.6 % for Grafix™ (p > 0.05), which was not statistically significant, and 10.2% for the untreated saline control group (p < 0.05); (ii) a 43.4% reduction in wound area four days after the second administration (day 8) of CYWC628 as compared to 37.6 % for Grafix™ (p > 0.05), which was not statistically significant, and 21.7% for the untreated saline control group (p < 0.05); (iii) a 69.3% reduction in wound area four days after the third administration (day 12) of CYWC628 as compared to 47.13% for Grafix™ (p < 0.05), which was statistically significant, and 36.4% for the untreated saline control group (p < 0.05), which was also statistically significant.; and (iv) an 83.8% reduction in wound area four days after the fourth administration (Day 19) of CYWC628 as compared to 66% for Grafix™ (p< 0.05), which was statistically significant, and 55.2% for the untreated saline control group (p<0.01), which was also statistically significant. Grafix™ results as compared to saline control were not statistically significant at any of the measured timepoints, whereas CYWC628 as compared to saline control was statistically significant at all measured timepoints.
The following graph and chart summarize the results of our Phase 5 pre-clinical study.
Effective wound healing is not only determined by the efficiency of wound closure, but also by the quality of the healed wound. For our multiple CYWC628 administration study, we also looked at several metrics essential to the quality of wound healing. These metrics are re-epithelialization, granulation, cell proliferation, neo-vascularization, and fibroblast recruitment. The results of the study indicated that at day 19 after the final treatment, CYWC628 had a significantly improved epithelization, granulation, cell proliferation (as measured using Ki67), neo-vascularization (as measured by CD31 and VEGF), and fibroblast recruitment (as measured by αSMA and IL-6) compared to control and Grafix™.
For our remaining pre-clinical studies, we will investigate multiple administrations of CYWC628 on a chemically induced chronic wound NONcNZO10/LtJ mouse model, complete a dose titration study to provide information on effective dose range of CYWC628, and complete an acute and chronic toxicity study. We expect to complete these studies in the 3rd quarter of 2024. Based upon our results achieved to date and the expected timing of these additional pre-clinical studies, we are planning to initiate a Phase 1/2 clinical trial in Australia for treatment of diabetic foot ulcers in 2025 with results expected in the third quarter of 2025.
Manufacturing: We are planning to complete a technology transfer of our cell manufacturing processes to a contract development and manufacturing organization, or CDMO, and conduct feasibility studies for our fibroblast spheroid-based drug product, with the intent to enter into a master services agreement with that CDMO to supply drug product for clinical trials. We expect to produce a master cell bank, working cell bank, and drug product for use in clinical trials by year end 2024.
| 3 |